Perfluoroalkyl and polyfluoroalkyl substances (PFAS) have won the title of "permanent chemicals" because they can exist in the environment for a long time. Now, they are increasingly proved to pose a serious risk to human health. In view of this increased awareness, scientists are stepping up efforts to better decompose them before they cause harm. A new breakthrough demonstrates how ultraviolet light can be used to do the job in a few hours.
PFAS is a group of chemicals composed of more than 4000 compounds, from waterproof clothing and non stick pots to food packaging and fire foam, which have their own characteristics. Widely used since the 1940s, studies have shown that the use of these chemicals is associated with a wide range of health conditions, including cancer and impaired immune system function.
In the ongoing efforts to eliminate chemicals from the environment, we have seen some promising progress. One of the 2020 studies shows that a new powder can be used as a catalyst to destroy some of the most stubborn forms of PFAS. Earlier this year, an interesting and landmark study found that through regular blood donation, the level of toxic PFAS chemicals in the blood could be reduced by 30%.
The latest discovery in this field comes from scientists at the University of California Riverside, who began to try to decompose PFAS chemicals through photochemical reaction in 2017. Adding these substances to water treatment reactors containing sulfite and exposing them to ultraviolet light has been shown to effectively decompose them. However, the chemical reaction is very slow, so the process uses a lot of energy and fails to disassemble all the strong fluorocarbon bonds, which are the basis of the long-term existence of PFAS chemicals.
In trying to improve this method, scientists have been adjusting the process and achieved some success last year. They found that the oxidation treatment before and after these baths led to 100% destruction of the carbon fluorine bond in the main PFAS pollutants. The team has now added a special component to this foundation to speed up development.
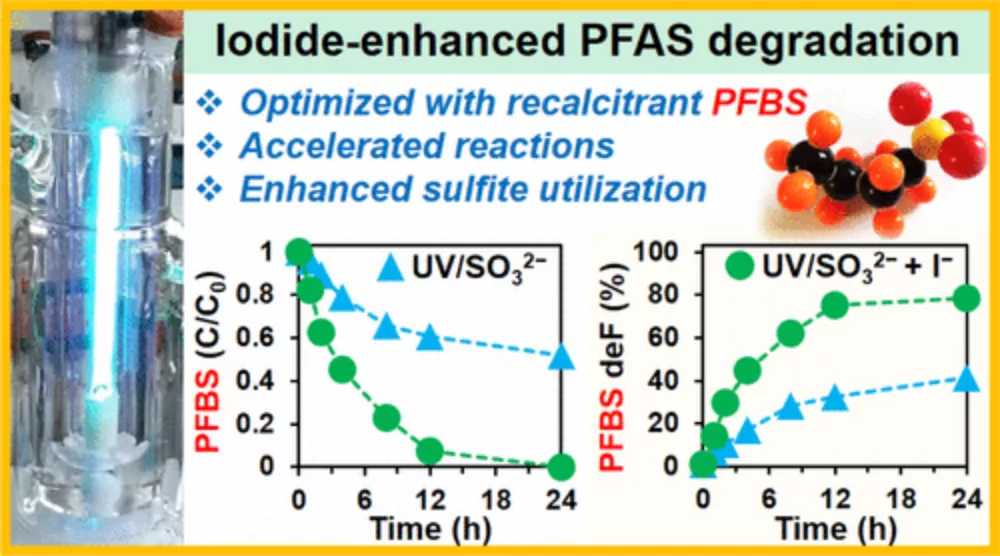
The addition of iodide to UV and sulfite treatment brings significant performance advantages. It can easily decompose common forms of PFAS. In addition, PFAS can be concentrated in brine solution, which is a promising sign of groundwater purification. The formula has also been proved to effectively decompose a particularly problematic four carbon PFAS molecule, Perfluorobutane sulfonate, which is completely removed from the solution within 24 hours.
Jinyong Liu, the corresponding author of the study and assistant professor of chemical and environmental engineering, said: "iodide is really doing some substantive work. It not only speeds up the reaction speed, but also allows the treatment of more than 10 times the concentration of PFAS, and even some very difficult structures."
According to the research team, in general, the team's new method has been found to destroy 90% of carbon and fluorine atoms in PFAS, which is faster than simple ultraviolet and sulfite treatment; Twice -- in a few hours. Scientists are now working with partners to pilot the technology.