
Platinum is a powerful catalyst for many different reactions and is most commonly used in fuel cells and catalytic converters for purifying automotive emissions. The problem, however, is that it is one of the rarest and most expensive metals on earth, limiting the scale of equipment using platinum catalysts. As a result, scientists have been experimenting with new methods to reduce the amount of platinum required for these devices, or even eliminate it altogether.
Now, researchers in Australia have found a new way to significantly reduce the amount of platinum required for these reactions. Instead of anchoring platinum atoms to a solid matrix, the team found a way to suspend them in a liquid, making them more effective and reusable.
First, platinum is dissolved in gallium at a temperature of about 300 ° C for several hours. Then, once the mixture cools down, it becomes a catalytic material and remains liquid above the low melting point of gallium 29.8 ° C. This is well below the usual melting point of platinum, 1768 ℃.
The resulting material has some amazing properties. It can carry out oxidation and reduction reactions, that is, adding or removing oxygen from substances. Even if Platinum only accounts for 0.0001% atoms in the alloy, the efficiency of this liquid catalyst is more than 1000 times higher than that of the solid catalyst composed of 10% platinum. Even better, by-products do not accumulate on the catalyst and reduce its effectiveness like solid catalysts.
When examining the liquid catalyst carefully, the team found that no two platinum atoms were in contact with each other - they were still dispersed in gallium. Curiously, platinum seems to be exerting influence on the surrounding gallium, which is doing catalytic work.
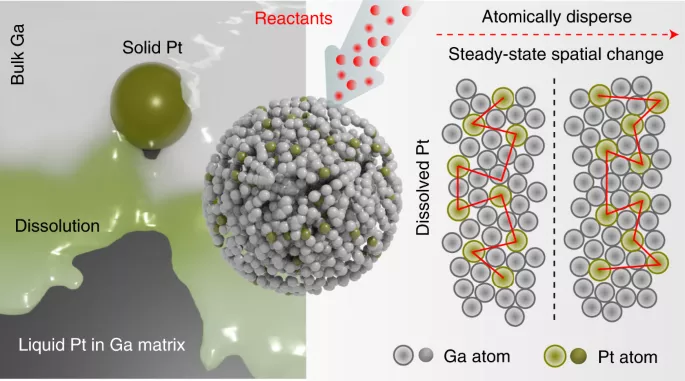
Dr. Andrew christofferson, the author of the study, said: "platinum is actually just a little below the surface, and it is activating the gallium atoms around it. Therefore, under the influence of platinum, magical things are happening on gallium. But without platinum, it would not happen. As far as I know, this is completely different from any other catalysis shown by anyone."
The team said that the new technology demonstrated a way to further expand our scarce platinum supply, allowing fewer quantities to achieve the same or even stronger catalytic effect. This should also reduce costs and make effective catalysts more widely used.
But the benefit is not just platinum -- the team says the liquid metal catalyst can combine with more than 1000 other elements to produce the same number of different reactions. Investigating these will be a focus of future work.
The study was published in natural chemistry 》In magazines.