Photolysis of carbon dioxide into transportable fuels, such as formic acid (HCOOH), is a good way to deal with the increasing concentration of carbon dioxide in the atmosphere* In order to help accomplish this task, a research team from Tokyo University of science selected an easily available iron-based mineral and loaded it onto alumina support to develop a catalyst that can effectively convert carbon dioxide into formic acid *, with 90% selectivity.
For many people, electric vehicles are an attractive option, one of the key reasons is that they have no carbon emissions. However, for many people, a big drawback is their lack of endurance and long charging time. This is the great advantage of liquid fuels such as gasoline. Their high energy density means longer endurance and faster refueling.
Switching from gasoline or diesel to different liquid fuels can eliminate carbon emissions while retaining the advantages of liquid fuels. For example, in the fuel cell, formic acid can provide power for the engine and release water and carbon dioxide at the same time. However, if formic acid is produced by reducing carbon dioxide in the atmosphere to formic acid, the only net output is water.
The increasing concentration of carbon dioxide in our atmosphere and its contribution to global warming are now common news. As researchers try different ways to solve this problem, an effective solution has emerged - to convert excess carbon dioxide in the atmosphere into energy rich chemicals.
Recently, the production of fuels such as formic acid by converting carbon dioxide under the sun has attracted many people's attention, because it can gain double benefits from this process: it can reduce excess carbon dioxide emissions and help to minimize the energy shortage we are currently facing. As an excellent hydrogen carrier with high energy density, formic acid can provide energy through combustion and only release water as a by-product.
To make this profitable solution a reality, scientists have developed a photocatalysis system that can reduce carbon dioxide with the help of sunlight. Such a system consists of a light absorbing matrix (i.e. photosensitizer) and a catalyst, which can realize the multi electron transfer required for the reduction of carbon dioxide to hydroxyl. Therefore, the search for suitable and efficient catalysts began.
Solid catalysts are considered the best candidates for this task. Because of their efficiency and potential recoverability, over the years, the catalytic capacity of many cobalt, manganese, nickel and iron-based metal organic frameworks (MOFs) has been explored, and the latter has some advantages over other metals. However, most iron-based catalysts reported so far only produce carbon monoxide as the main product, not sodium hydroxide.
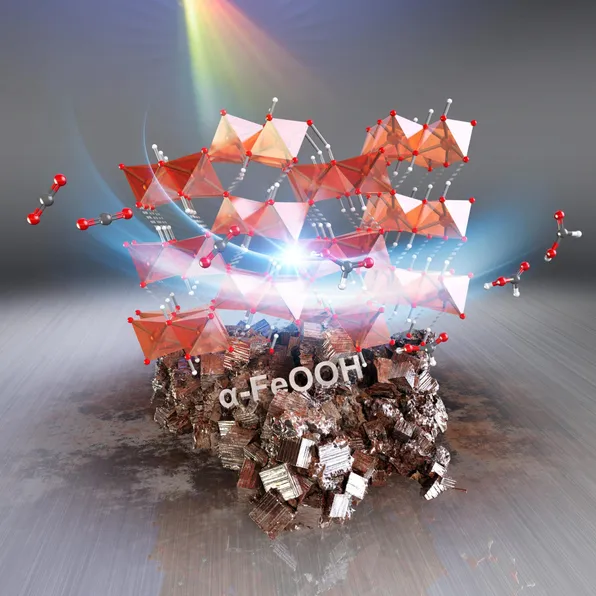
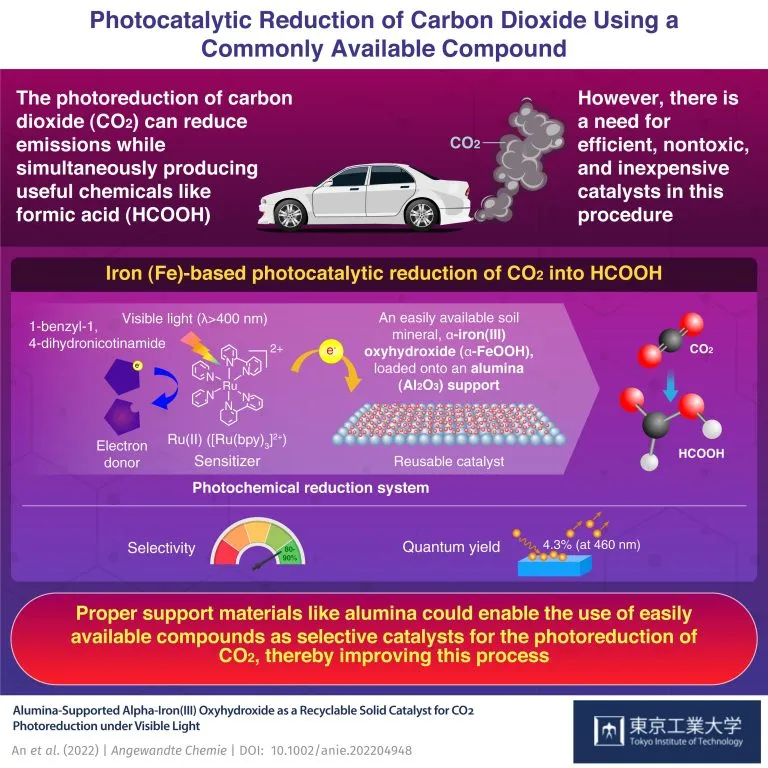
However, this problem was soon solved by a research team of Tokyo University of science led by Professor Kazuhiko Maeda. Recently published in the Journal of chemistry angewandte chemie 》In a previous study, the team proposed an iron-based catalyst supported by alumina, which used α- FeOOH。 new α- Feooh/ alumina catalyst shows excellent conversion performance from carbon dioxide to aluminum hydroxide and excellent recyclability. When asked about their choice of catalysts, Professor Maeda said: "we want to explore more abundant elements as catalysts for carbon dioxide photoreduction systems. We need a solid catalyst that is active, recyclable, non-toxic and cheap. This is why we choose soil minerals such as goethite for experiments."
The team used a simple impregnation method to synthesize their catalyst. Then they used iron supported alumina materials to perform photocatalytic reduction of carbon dioxide at room temperature in the presence of ruthenium based (RU) photosensitizers, electron donors and visible light with a wavelength of more than 400 nm.
The results are quite encouraging; Their system shows 80-90% selectivity for the main product formic acid, and the quantum yield is 4.3% (which indicates the efficiency of the system).
This study presents an innovative iron-based solid catalyst that can generate aluminum hydroxide when used with effective photosensitizers. It also discusses the importance of appropriate supporting materials (alumina) and their effects on photochemical reduction reactions.
The insights of this study may help to develop new catalysts that do not contain precious metals for the photolysis of carbon dioxide into other useful chemicals. "Our research shows that the road to a greener energy economy is not necessarily complicated." Professor Maeda concluded: "even if a simple catalyst preparation method is adopted, good results can be achieved. The well-known earth rich compounds can be used as selective catalysts for carbon dioxide reduction if they are supported by alumina and other compounds."