A recently developed catalyst for decomposing plastics is promoting the upgrading of plastics A team of scientists led by scientists from Ames laboratory discovered the first process inorganic catalyst in 2020 to decompose polyolefin plastics into molecules that can be used to make more valuable products. The team has now developed and validated a strategy to accelerate conversion without sacrificing the desired product.
The catalyst was originally designed by huangwenyu, a scientist at Ames laboratory. It consists of platinum particles supported on a solid silica core surrounded by a silica shell with uniform pores, which provides access to the catalytic point. The total amount of platinum required is quite small, which is important because of the high cost and limited supply of platinum. In the deconstruction experiment, the long polymer chain penetrates into the pores and contacts the catalytic sites, and then the chain is decomposed into smaller fragments, which are no longer plastic materials.
According to Aaron Sadow, a scientist at Ames lab and director of icoup, the team has made three different catalysts. Each variant has the same size core and porous shell, but the platinum particles have different diameters, ranging from 1.7 to 2.9 to 5.0 nm.
The researchers assume that different platinum particle sizes will affect the length of the product chain, so large platinum particles will produce longer chains, and small platinum particles will produce shorter chains. However, the team found that the product chain length of all three catalysts was the same.
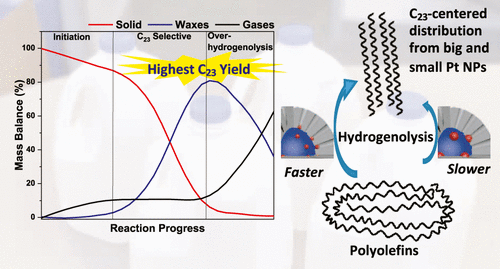
"In the literature, the selectivity of carbon carbon bond cleavage reaction usually varies with the size of platinum nanoparticles." "By placing platinum at the bottom of the pores, we see something quite unique," Sadow said
On the contrary, the speed at which the chains of the three catalysts are broken down into small molecules is different. Larger platinum particles react more slowly with long polymer chains, while smaller platinum particles react faster. This increase in velocity may be due to the higher proportion of platinum sites at the edges and corners of the surface of smaller nanoparticles. These sites are more active in cracking polymer chains than platinum on the surface of particles.
According to Sadow, these results are important because they show that the activity can be adjusted independently of the selectivity of these reactions. "Now, we are confident that we can produce a more active catalyst that can chew the polymer faster and use the catalyst structure parameters to shift the specific product chain length," he said
Huang Wenyu explained that in general, this kind of macromolecular reactivity has not been widely studied in porous catalysts. Therefore, this research is very important to understand the basic science and its performance in plastic upgrading and recycling.
"We really need to learn more about this system because we are learning new things every day. We are exploring other parameters that can be adjusted to further improve production speed and change product distribution," said Huang. "So, in our list, there are many new things waiting for us to discover."