*The researchers proposed the first graphene synthesis technology using carbon monoxide as a carbon source. This is a fast and cheap method, which can produce high-quality graphene * with relatively simple equipment for electronic circuits, gas sensors, optics and other fields. The research was carried out by scientists from skoltech, MIPT, the Institute of solid state physics of the Russian Academy of Sciences, the University of Alto and other institutions. The research has been published in the famous advanced science 》Published in a magazine.

Chemical vapor deposition (CVD) is a standard technology for the synthesis of graphene. Graphene is an atom thick sheet composed of carbon atoms arranged in a honeycomb. It has unparalleled characteristics and can be used in electronic applications. CVD usually involves the separation of carbon atoms from gas molecules and precipitation on the substrate in a single layer in a vacuum chamber. Copper is a popular base material, and the gases used have always been hydrocarbons: methane, propane, acetylene, spirits, etc.
"The idea of synthesizing graphene from carbon monoxide has existed for a long time, because this gas is one of the most convenient carbon sources for the growth of single-walled carbon nanotubes. We have nearly 20 years of working experience in carbon monoxide. However, our first experiment using graphene was not successful, and it took us a long time to understand how to control the nucleation and growth of graphene." "The main attraction of this single-layer graphene is that it can decompose completely under the pressure of our researchers," said Professor skbulin
"This project is one of the outstanding examples of how basic research can benefit applied technology." Dmitry krasnikov, the co-author of the paper and senior research scientist of skoltech company, stressed: "due to the understanding of the deep dynamic mechanism of graphene formation and growth verified by theory and experiment, the optimal conditions for the formation of large graphene crystals become feasible."
This new approach benefits from the so-called self limiting principle. At high temperatures, carbon monoxide molecules tend to decompose into carbon and oxygen atoms when they are close to the copper substrate. However, once the first layer of crystalline carbon is deposited and separates the gas from the substrate, this trend will subside, so this process is naturally conducive to the formation of a single layer. Methane based CVD can also operate in a self limiting manner, but to a lesser extent.
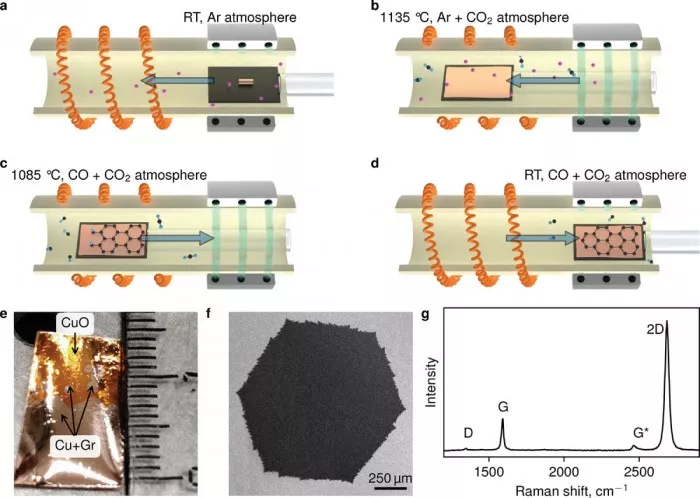
"The system we use has many advantages. The resulting graphene is purer, grows faster and forms better crystals. In addition, this adjustment prevents accidents by completely eliminating hydrogen and other explosive gases in the process," said the study's lead author, skoltech Intern Artem grebenko.
The fact that this method eliminates the risk of combustion means that no vacuum is required. The equipment works under standard pressure, which makes it much simpler than traditional CVD equipment. Simplified design in turn leads to faster synthesis. "It takes only 30 minutes from taking a piece of bare copper to pulling out graphene," grebenko said
Since vacuum is no longer needed, the equipment not only works faster, but also becomes cheaper. "Once you give up the high-end hardware that produces ultra-high vacuum, you can actually assemble our 'garage solution' for no more than $1000," the researchers stressed
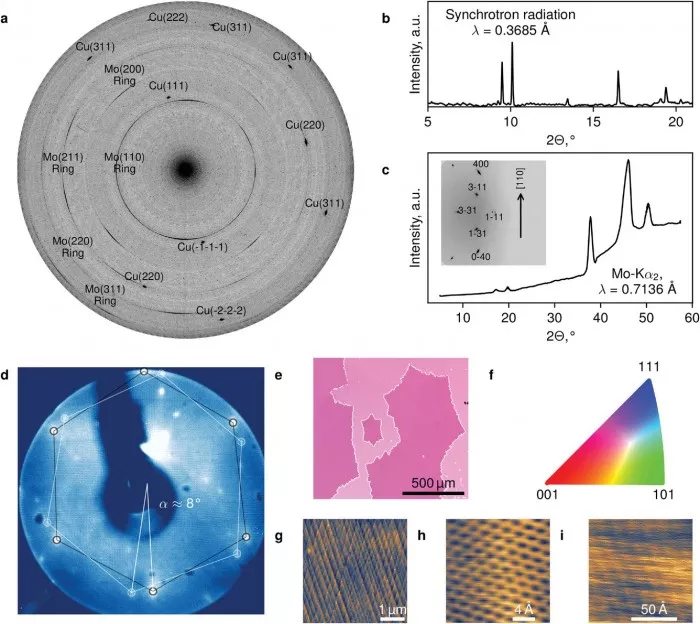
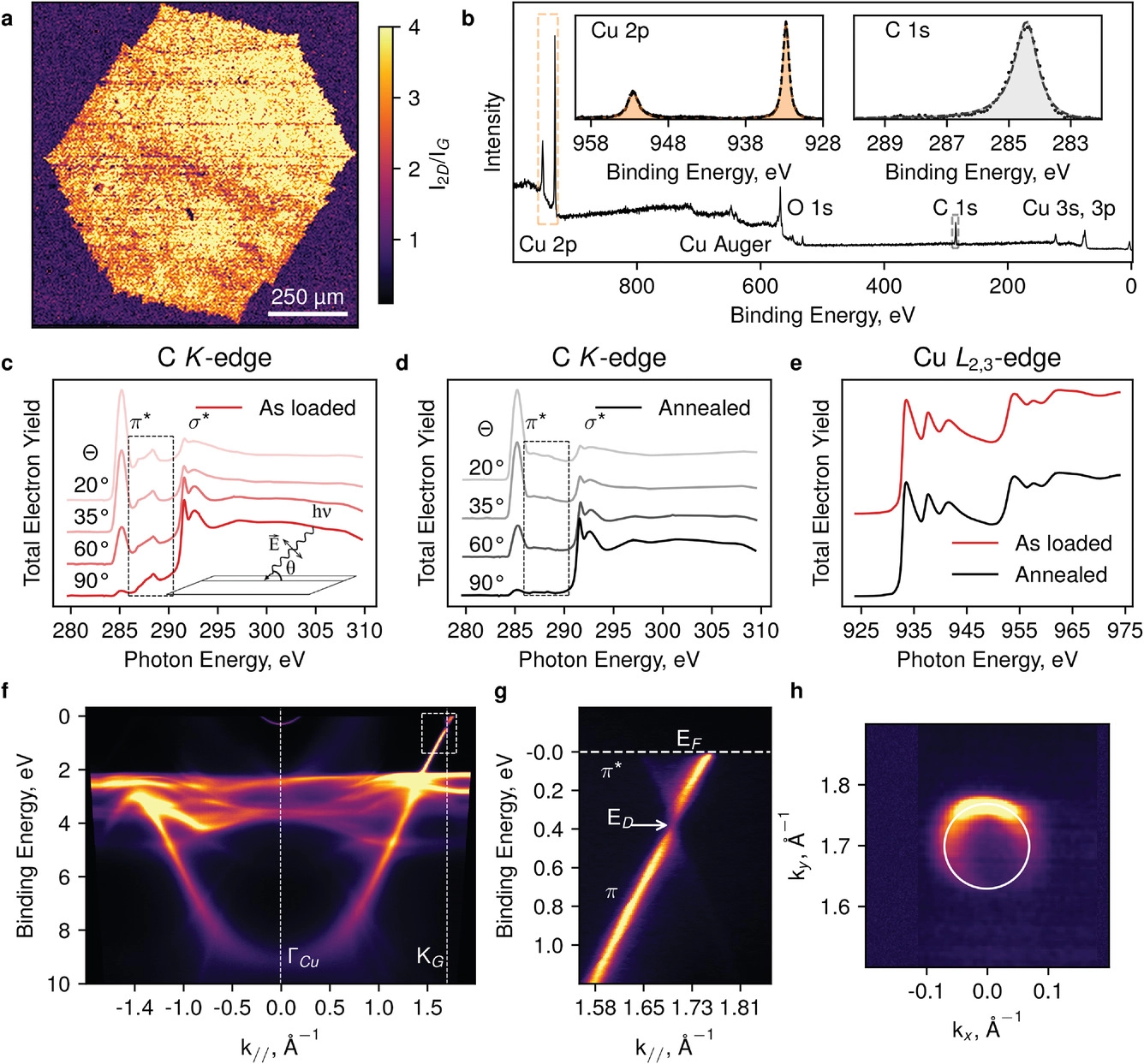
Boris gorshunov, co-author of the study and professor of MIPT, stressed the high quality of the materials produced. "Whenever a new graphene synthesis technology is proposed, researchers must prove that it can produce the effect they claim. After rigorous testing, we can confidently say that our graphene is indeed high-grade and can compete with materials produced by other gases through CVD. The resulting materials are crystalline, pure and have enough fragments to be used in electronic products."
In addition to the standard application of graphene itself, there is also an intriguing possibility to use graphene bound to copper without removing metal. Compared with methane, carbon monoxide has very high adhesion energy to metals. This means that with the occurrence of deposition, graphene can not only protect the copper layer from chemical reactions, but also endow it with structure, creating a highly developed metal surface with great catalytic performance. Other metals, such as ruthenium and palladium, will also play a role in this case, opening up a way for new materials with unusual surfaces.