By storing energy through rechargeable battery technology, our digital lifestyle is full of power. On the one hand, renewable energy can be incorporated into the power grid. However, the function of the battery in cold conditions is still a challenge, prompting people to study and improve the low-temperature performance of the battery. The discharge rate of aqueous battery (in liquid solution) at low temperature (measuring the energy released per unit time) is better than that of non-aqueous battery.
A new study by engineers of the University of Hong Kong was recently published in the journal nano research energy, which proposed the best design elements of aqueous electrolyte for low-temperature aqueous batteries. The study examined the physicochemical properties of water electrolytes (which determine their performance in the battery) according to several indicators: phase diagram, ion diffusivity and kinetics of redox reaction.
The main challenge of low temperature aqueous battery is that the electrolyte freezes, the ion diffusion is slow, and the redox kinetics (electron transfer process) is slow. These parameters are closely related to the physicochemical properties of low-temperature water-based electrolytes used in batteries.
Therefore, in order to improve the performance of the battery under cold conditions, it is necessary to understand the reaction of electrolyte to cold (-50 OC to -95 OC / -58 of to -139 of). Yi Chun Lu, the research author and associate professor, said: "in order to obtain high-performance low-temperature aqueous solution batteries (LT ABS), it is very important to study the physical and chemical characteristics of aqueous electrolyte changing with temperature to guide the design of low-temperature aqueous electrolyte (LT AES)."
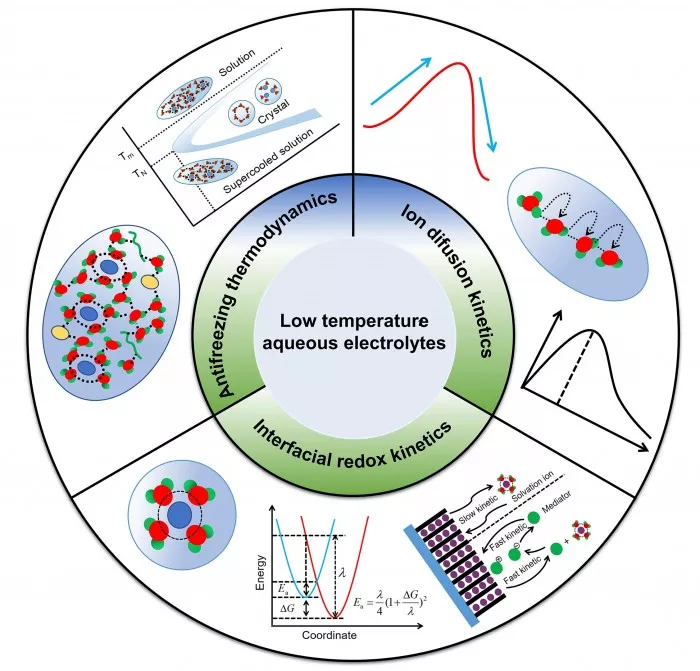
The figure shows the design strategy of water electrolyte, including antifreeze thermodynamics, ion diffusion kinetics and interfacial oxidation-reduction kinetics.
The researchers compared various lt-aes used in energy storage technology, including li+/na+/k+/h+/zn2+- battery, supercapacitor and mobile battery technology. The study collated information on the performance of various LT AES in many other reports, such as antifreeze hydrogel electrolytes for zn/mno2 water batteries; And ethylene glycol (eg) -h2o mixed electrolyte for Zn metal battery.
They systematically studied the equilibrium and nonequilibrium phase diagrams of these reported LT AES to understand their antifreeze mechanism. The phase diagram shows the change of electrolyte phase at different temperatures. The relationship between the conductivity of LT AES and temperature, electrolyte concentration and charge carrier was also investigated.
Lu, the author of the study, predicted that "the ideal antifreeze water electrolyte should not only exhibit a low freezing point temperature Tm, but also have a strong supercooling ability", that is, the liquid electrolyte medium should maintain a liquid state even when it is below the freezing point temperature, so as to realize ion transmission at ultra-low temperature.
The authors found that most of the LT AES that enable the battery to operate at ultra-low temperature show low freezing point and strong supercooling ability. In addition, "the strong supercooling ability can be achieved by increasing the minimum crystallization time t and the ratio of the glass transition temperature to the freezing temperature (tg/tm) of the electrolyte".
By reducing the energy required for ion transfer, adjusting the electrolyte concentration, and selecting some charge carriers that can promote the rapid redox reaction rate, the charge conductivity of the reported lt-ae for batteries can be improved. Lu said: "reducing the diffusion activation energy, optimizing the electrolyte concentration, selecting charge carriers with low hydration radius, and designing the cooperative diffusion mechanism will be effective strategies to improve the ionic conductivity of LT AES."
In the future, the author hopes to further study the physicochemical properties of electrolytes that can help to improve the performance of low-temperature underwater batteries. Lu said, "we hope to develop high-performance low-temperature water batteries (LT ABS) by designing water-based electrolytes with low freezing temperature, strong supercooling capacity, high ionic conductivity and fast interfacial redox kinetics."