Tesla's Canadian advanced battery research team, in cooperation with Dalhousie University, released a new paper. This paper introduces a new nickel based battery which can be used for 100 years, and its charging and energy density are better than lithium iron phosphate battery. Time goes back to 2016. This year, Tesla cooperated with Jeff Dahn battery Laboratory of Dalhousie University in Canada to establish the "Tesla advanced battery research" department.
Dahn is considered a pioneer in lithium ion batteries. Since the birth of lithium-ion battery, he has been engaged in research in this field and made great achievements in extending the battery life cycle, which is very important for the commercialization of battery.

Dahn's current work mainly focuses on improving the energy density, durability and cost reduction of the battery. His team has contributed quite a number of battery patents and papers to Tesla. In 2019, his team published a paper saying that the new battery belongs to a lithium-ion battery with a next-generation "single crystal" NMC cathode and a new advanced electrolyte. Based on extensive tests, they believe that the new battery can provide a endurance of "more than 1.6 million kilometers (1million miles)" for electric vehicles.
In 2021, Tesla renewed its contract with the team for five years and added two new leaders, led by Dahn.
Recently, Michael Metzger, one of the two leaders, together with Dahn and several other doctors, published a new paper entitled li[ni0.5mn0.3co0.2]o2 as a superior alternative to LiFePO4 for long lived low voltage Li ion cells in the Journal of the electrical society.

This paper mainly describes a variant of the traditional nickel manganese cobalt battery (nmc532). The difference from similar batteries is that it is optimized to operate at 3.8V, while other NMC batteries use 4.2V or higher. The lower voltage greatly extended the battery life, which led Dahn and his team to propose that the battery could have a service life of 100 years. This allows the nmc532 battery to compete with the lithium iron phosphate battery (LPF) in life, while retaining other popular features, such as higher energy density, which allows electric vehicles to travel longer distances with fewer batteries.
The team wrote in the abstract of the paper:
The graphite contained is only enough to charge the single crystal li[ni0.5mn0.3co0.2]o2//graphite (nmc532) soft pack battery operating at 3.8V to 3.65 V or 3.80 V, so as to compare it with lifepo4//graphite (LFP) soft pack battery based on similar maximum charging potential and negative electrode utilization. When the nmc532 battery is only composed of graphite enough to charge to 3.80 V, its energy density is higher than that of LFP battery at 40 ° C, 55 ° C and 70 ° C, and its cycle life is much longer than that of LFP battery. The electrolyte containing lithium difluorosulfimide (lifsi) salt shows excellent service life at high temperature, which is far more than the traditional LiPF6 electrolyte.
The capacity retention capability of the battery is impressive in a high number of cycles:
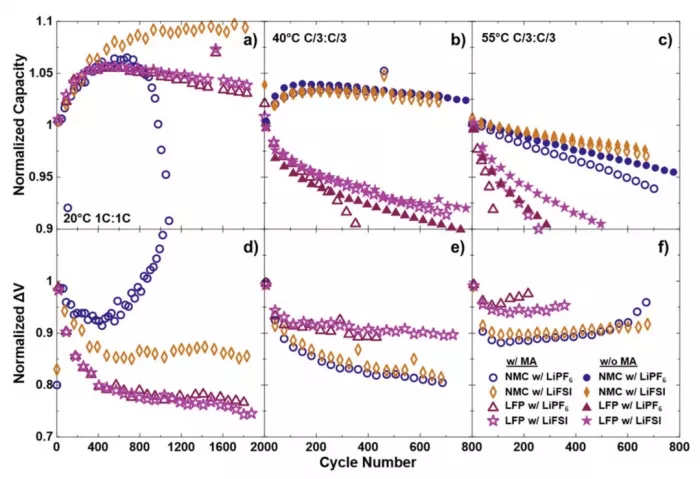
The research team even pointed out that if the temperature is controlled at 25 ° C, the battery described in the paper can be used for 100 years:
Ultra-high precision coulometric analysis and electrochemical impedance spectroscopy were used to supplement the cycle results, and the reasons for the improvement of NMC battery performance were studied. Compared with LFP batteries, NMC batteries, especially those balanced and charged to 3.8 V, show better coulomb efficiency, less capacity attenuation and higher energy density, and are expected to have a life of nearly one century at 25 ℃.
One of the keys seems to be the use of lifsi lithium electrolyte.
So when will Tesla put this battery into mass production vehicles? Probably never. The researchers said that their new batteries cost more than LFP batteries and may not have the power characteristics required by electric vehicles. But they may be well suited for long-term energy storage. If so, the initially higher costs will be offset by a significantly longer service life. Of course, the disadvantage is that new batteries continue to use increasingly expensive raw materials such as nickel and cobalt, and there are some social disputes about cobalt mining.
However, the paper points out that the benefits they have found can also be applied to chemicals that are cobalt free or low.