Bacteria may be very good at developing resistance to our best drugs, but there are some things they can't evolve their own way -- like tiny drill bits tearing them apart Rice University researchers are now showing off their next generation of molecular drills that can be activated by blue light and could revolutionize the fight against superbugs
Bacteria quickly adapt to changes in their environment, which unfortunately includes antibiotics. Some bacteria naturally mutate, allowing them to survive attacks and pass these genes on to their offspring. Over time, this resistance has accumulated to the point that most of our once powerful antibiotics have failed, which may eventually lead to a "new dark era of medicine", leading to the basic infection becoming fatal again.
But while bacteria can develop resistance to chemical attack, they cannot defend against physical attack. Therefore, scientists are studying the use of serrated surfaces, black phosphorus coatings, liquid metal crushers, miniature traps, "poison arrows" and molecular drills to kill superbacteria.
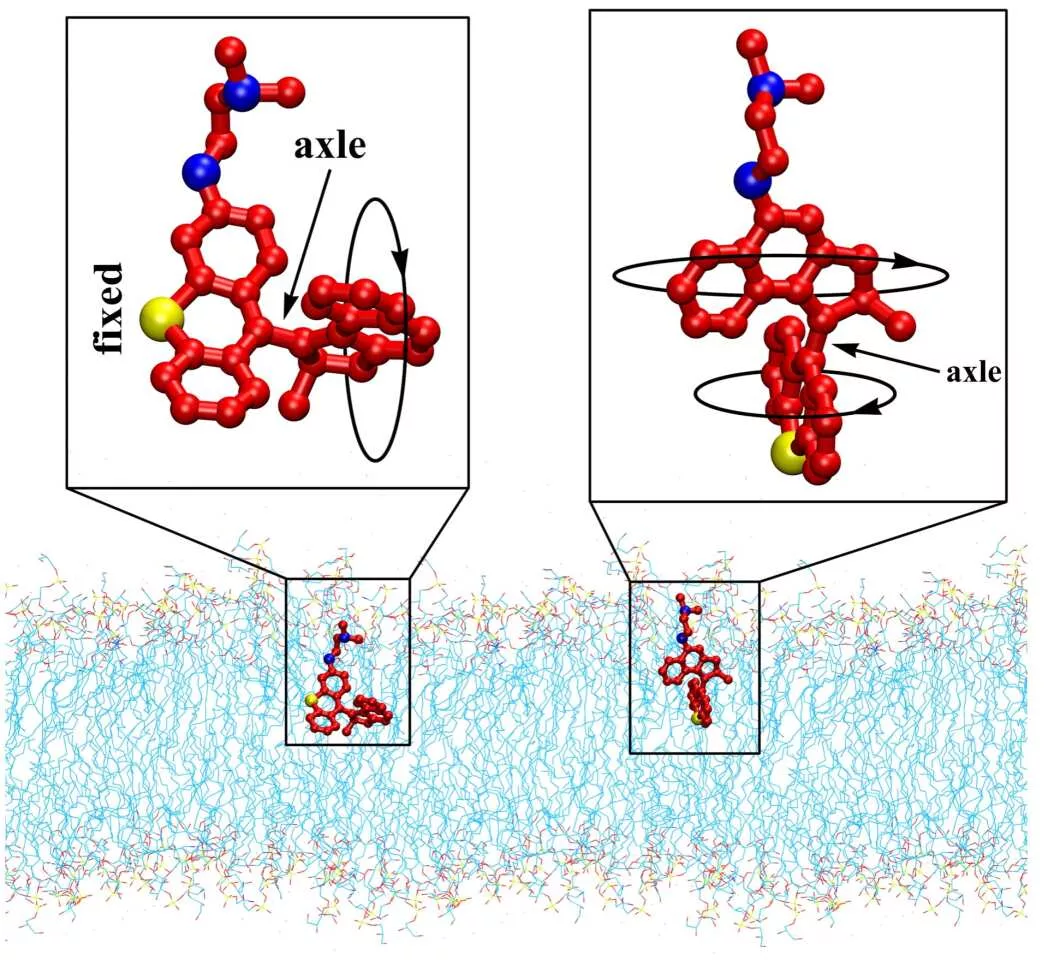
Rice University's team is now working on the last basis. These molecules are designed to have moving rotors that rotate 2million to 3million times per second when activated by light, making them effective drill bits. When this movement is directed at bacteria, it can pierce their outer membranes and overflow their interiors - either killing the cells completely or providing an opening for antibiotics to enter and complete their work.
Now, Rice University researchers have made some adjustments to these bits. The previous iteration was activated by ultraviolet light, but now the new iteration is run by blue light, which is harmless to human cells and already has some antibacterial properties. The team also made some modifications to better guide the drill bit towards the bacteria by positioning the negative charge of the bacterial membrane.
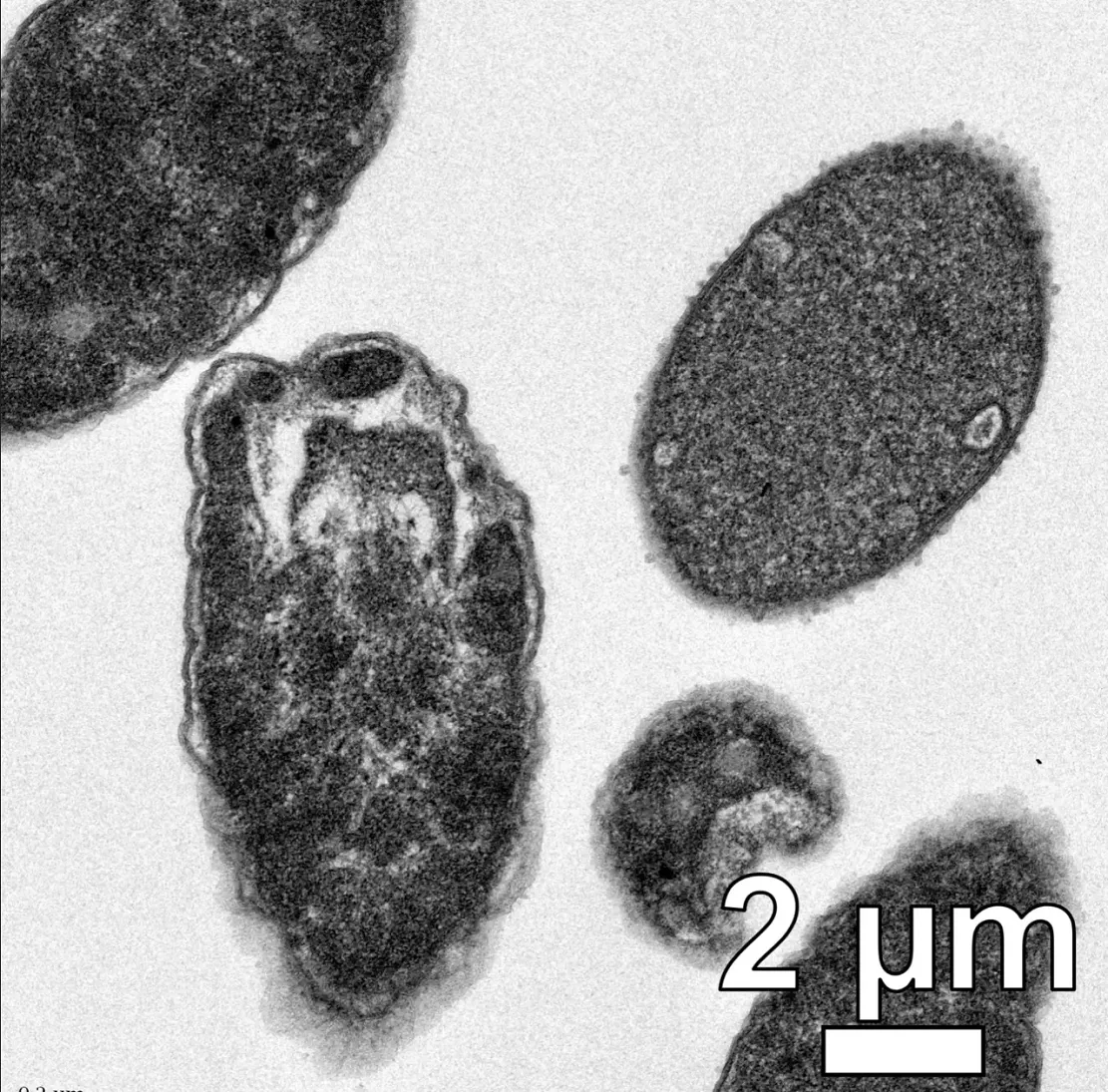
The team tested six different drill bits and found that all of them could drill holes in the membranes of two major types of bacteria - gram negative and gram positive - and reduce their levels to below detectable levels within minutes. These drills can also effectively break through biofilms -- the protective shields that bacteria build around their colonies -- and kill persistent cells that survive by resting in antibiotics.
Although the drill alone can kill most bacteria, the addition of antibiotics makes the treatment more powerful. Even if these bacteria have developed resistance to these specific drugs, this is effective because it seems that the drill bit can help bypass the evolutionary defense system. This means that the drill may not only be a promising new therapy in itself, but also extend the service life of existing antibiotics.
The team said that what they need to do next is to connect the bacterial specific peptide label with the drill bit, which will help them hunt the target strain more accurately without affecting human cells.