A human trial showed that hydrogel injection is a promising and effective treatment for chronic low back pain caused by intervertebral disc degeneration (DDD) This treatment is much less traumatic than other operations and has been approved by the U.S. Food and Drug Administration (FDA).
Intervertebral discs play an important role in buffering the vertebral body, but like many parts of the body, they begin to wear out with age. In many people, the liquid filling of the intervertebral disc dries up or leaks, causing pain and impairing mobility. Unfortunately, the treatment methods are mainly limited to rest, physical therapy, painkillers and other care, or in more serious cases, surgical discectomy or prosthesis replacement.
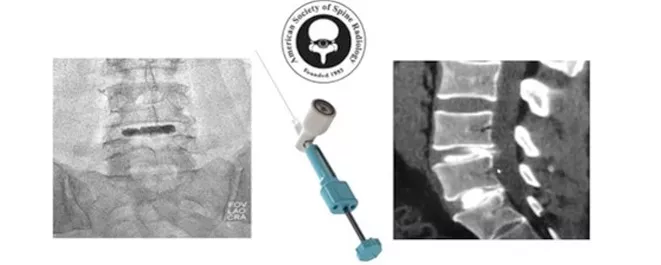
But recently, scientists have developed a new option -- a hydrogel that can be injected into the spine. It can fill the cracks and tears of the affected intervertebral disc and restore its partial cushioning effect to reduce pain. This gel was developed by the medical company regeltec, called hydrafil, and obtained the breakthrough device certification from FDA in 2020. Now, the results of hydrafil's first human trial have been released.
The trial involved 20 patients aged between 22 and 69 who had chronic low back pain caused by DDD. They all described their pain as 4 or higher on a 10 point scale, and all reported that existing therapies provided only mild relief.
First, the gel was heated to make it a viscous liquid, and then injected into the affected intervertebral disc with a No. 17 needle. When it cools to body temperature, it will form an implant with the same biomechanical properties as the natural intervertebral disc. The researchers then followed the patients for six months to assess their recovery.
Sure enough, all participants reported improvements in pain and mobility. In the range of 0 to 10, the reported mean pain level decreased from 7.1 to 2.0. In a questionnaire on how their lower back pain interfered with their daily activities, their average score fell from 48 to 6.
Douglas beall, the study's lead author, said: "if these findings are confirmed in further studies, this may be a very promising treatment for chronic low back pain that has not been fully relieved from conservative treatment. This gel is easy to manage, does not need surgery, and is a simple procedure for patients."
Of course, the scale of this first trial is very small, and 20 patients are not enough to make any big claims. But this treatment does show promise, and the team will continue to test more patients.
The study will be presented at the Interventional Radiology Association's annual scientific meeting next week.